ISO 13485 Certification
🧬 ISO 13485 Certification in 30 Days*
🏥 Build Quality & Compliance in Medical Devices
- Ensure product safety and regulatory compliance
- Improve process consistency and risk management
- Gain trust of healthcare providers and customers
- Meet global market entry requirements
- 100 % guarantee — we don’t get paid until you’re certified
🎁 Limited Time: Get a Free 2-Hour Training & Awareness Session with Certificates — for a Group of 5!
🧬 Benefits of ISO 13485 Certification
🏥 Product Safety
Ensures consistent quality and performance of medical devices.
⚖️ Regulatory Compliance
Meets strict international standards for medical-device production.
⚙️ Process Control
Improves traceability, production efficiency, and device reliability.
🧩 Risk Reduction
Identifies and mitigates defects and hazards in manufacturing.
🤝 Customer Confidence
Builds trust among healthcare professionals and patients.
🏆 Global Market Access
Enables compliance for entry into international healthcare markets.
ISO 13485:2016 is the quality standard stating the requirements of the Quality Management System (QMS) for the design and manufacture of Medical Devices.
ISO 13485 is a recognized international standard for quality management systems in the medical device business. It assures that organizations involved in the design, development, manufacturing, storage, distribution, installation, and servicing of medical devices comply with regulatory requirements and maintain the highest quality standards.
The organizations that are involved in the manufacturing and handling of medical devices are required to adhere to the norms of ISO 13485 medical devices standard. ISO 13485 is a set of standards that helps in the implementation of the Quality Management System for Medical Devices (MD-QMS).
It demonstrates the competency of the organizations in delivering good quality and safe medical devices and relevant medical services that can fulfill the customer’s requirements as well as ensure compliance with the regulatory norms. The latest version of ISO 13485 Certification was published in 2016 and hence, it is termed ISO 13485:2016.
ISO 13485 certification involves building a quality management system for medical devices by identifying potential risks and documenting them effectively. The threats may arise from contamination of equipment or errors during handling. ISO 13485 provides for analyzing those threats and planning appropriate actions to prevent those risks.
Our vast network of experienced auditors all over the world helps you achieve this certification in a time-bound and hassle-free manner.
📑 Table of Contents – ISO 13485 Certification
What are the Principles of ISO 13485 Certifications?
Customer focus – aiming to improve for the betterment of the interested parties and customers, this will help one sustain customers, increase customer base, and make sure to communicate their needs and expectations by monitoring throughout the organization.
Leadership – to achieve quality objectives leaders need to establish unity of purpose which is by aligning their strategy, policies, procedure, and resources this will lead to better coordination of the organization’s processes one needs to establish a culture of trust and integrity, provide people with the required resource, training, authority to act with accountability.
Engagement of people – for efficiency involves people of all levels, this can be done by communicating with the employees their needs in the organization, sharing knowledge, and experience, and recognizing people’s contribution, learning, and improvement.
Process approach – when activities are understood and then executed then the efficiency of the delivered output will increase, by understanding organizations’ capabilities and determining resource constraints prior to action.
Improvement – improvement is important for an organization to maintain the current level of performance and to even keep on developing, this can be done by giving proper training and letting them understand how work happens with that track, reviewing and audit planning, implementation, recognizing and acknowledgment, which will result into anticipation of internal and external risks and opportunity, improved process performance.
Evidence – based decision making – learn from mistakes, it is simply that decisions should be driven by evaluation of data, this will help one take better efficient solutions adding more, intuitions should never be neglected.
Relationship management – manage relations with relevant interested parties such as providers, one can achieve this by keeping a well-managed supply chain that provides a stable flow of products and services, determining interested party relationship that needs to be managed.
Why is ISO 13485 Certification Important?
- Guarantees adherence to global regulatory standards, such as EU MDR and FDA criteria.
- Boosts customer trust by showcasing dedication to product excellence and ensuring patient safety.
- Facilitates market growth by satisfying conditions for global commerce.
- Mitigates risk through the application of organized quality management procedures.
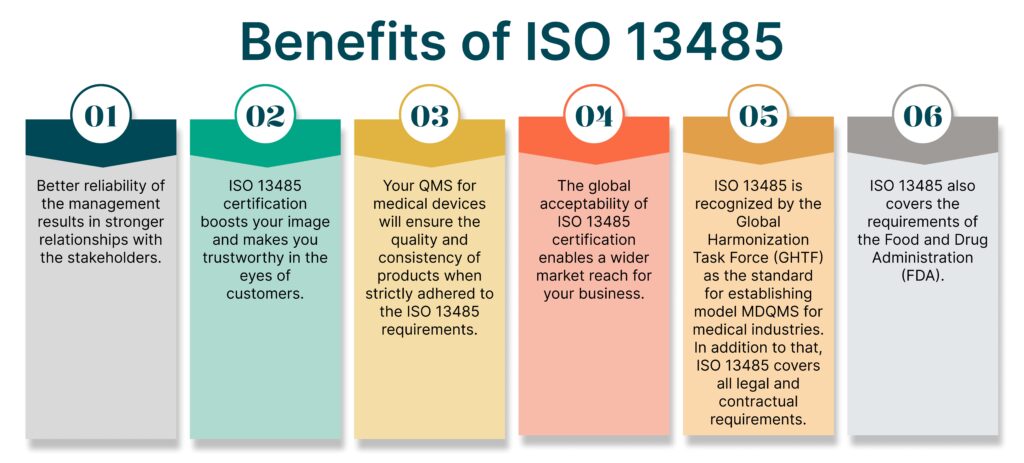
Benefits of ISO 13485
ISO 13485 is helpful for organizations in all stages of the product life cycle- starting from its design development, manufacturing, storage, and distribution of the final product. The applicability of ISO 13485 does not stop at that. It can also be applied for relevant services of medical devices along with associated activities that are in the form of technical support- both remote and on-site.
Since ISO 13485 is an internationally recognized standard, building your MDQMS in accordance with it has unfathomable benefits for your organization. It tremendously reduces your costs. Some of the many benefits of ISO 13485 certification are listed below:
Scope of ISO 13485 Certification
Non-Active Medical Devices (A.1)
Initially, the MD-QMS accreditation covered one Main Technical Area (MTA), focusing on non-active medical devices. This includes:
- A.1.1 General Non-Active, Non-Implantable Medical Devices – Devices that do not require power to function, such as bandages and surgical instruments.
- A.1.2 Non-Active Implants – Medical implants such as orthopedic screws, plates, and dental implants.
- A.1.3 Devices for Wound Care – Products like dressings and wound-closure strips that aid in the healing process.
- A.1.4 Non-Active Dental Devices and Accessories – Tools and accessories used in dental treatments.
- A.1.5 Other Non-Active Medical Devices – Includes a range of additional non-powered medical devices.
In Vitro Diagnostic (IVD) Medical Devices (A.4)
With the 2024-25 surveillance audit, three more MTAs were added, including IVD medical devices. These include:
➤ A.4.1 Reagents & Reagent Products, Calibrators, and Control Materials for:
🢚 Clinical Chemistry
🢚 Immunochemistry (Immunology)
🢚 Hematology/Haemostasis/Immunohematology
🢚 Microbiology
🢚 Infectious Immunology
🢚 Histology/Cytology
🢚 Genetic Testing
➤ A.4.2 In Vitro Diagnostic Instruments & Software – Medical devices used in diagnostic testing and related software.
➤ A.4.3 Other IVD Medical Devices – Additional products that fall under the IVD category.
➤ Sterilization Methods for Medical Devices (A.5)
Ensuring medical devices are contamination-free is critical. ISO 13485 includes compliance with sterilization processes such as:
- A.5.1 Ethylene Oxide Gas Sterilization (EOG) – Used for heat-sensitive devices.
- A.5.2 Moist Heat – Common method using steam under pressure.
- A.5.3 Aseptic Processing – Ensures sterile environments during production.
- A.5.4 Radiation Sterilization – Uses gamma rays, e-beams, or X-rays.
- A.5.5 Other Sterilization Methods – Covers additional approved sterilization techniques.
➤ Distribution, Parts, and Services (A.7)
Medical device quality management extends beyond manufacturing. It covers supply chain aspects such as:
- A.7.1 Raw Materials – Ensuring the quality of materials used in production.
- A.7.2 Components – Verification of components for medical device assembly.
- A.7.3 Subassemblies – Ensuring subassembled products meet quality standards.
- A.7.4 Calibration Services – Regular calibration of medical devices and equipment.
- A.7.5 Distribution Services – Ensuring safe transportation and handling.
- A.7.6 Maintenance Services – Routine servicing of medical equipment.
- A.7.7 Transportation Services – Compliance in transporting medical devices securely.
- A.7.8 Other Services – Additional medical device-related services.
ISO 13485 Requirements
Define the scope – It is very important to understand the purpose and market of the medical device in order to define the scope. It is also important to consider the regional regulations related to the product.
Perform ISO 13485 Audit & analyze the gap – An audit validates the conformities of your management system against the requirements of the management system. Any gaps should be thoroughly examined.
Prepare a project plan – The gap analysis after the audit helps you in developing a corrective action plan that takes into consideration all the non-conformities and ensures that you are compliant with ISO 13485 requirements as well as with other regional regulations.
Maintain documentation – the mandatory documentation, i.e., Quality Manual, procedures, work instructions, and documentation with evidence of proof of implementation should be maintained for the ease of the audit.
Train your workforce – It is very important to educate your workforce involved in the production of medical devices, regarding the requirements of ISO 13485 medical devices certification. This includes both legal and procedural requirements.
Implementation – Once you have established your quality management system for medical devices, you must run it for a period of at least three months and document your processes.
Conduct an internal audit – This is necessary to check for any deviation from the ISO 13485 certification requirements. It can either be conducted by one of your qualified employees or by any third party.
Conduct a management review – A management review is necessary to ensure that your quality management system is functioning as per the requirements.
Apply for ISO certification – This involves inviting a certification body to conduct an audit on your management system. After the successful completion of the audit, you may be awarded ISO 13485 certification.
PDCA Cycle
- Plan – to think that what do we need to achieve in our organization
- Do – to execute a planned action which will help us achieve the required objective
- Check – monitor against the standards) (policies, objectives, requirements)
- Action – finally implementing what has been rechecked.
ISO 13485 Frequently Asked Questions (FAQs)
Answer:
ISO 13485 certification is a globally recognized standard for demonstrating the effectiveness of a Quality Management System (QMS) for medical devices. Its aim is to ensure the production of safe, reliable, and high-quality medical devices that meet customer requirements and strengthen customer trust.
Answer:
An accredited certification body conducts the external audit using defined procedures and checklists to verify that all ISO 13485 requirements have been properly implemented in your organization.
Answer:
The latest version is ISO 13485:2016, published in March 2016. It focuses on ensuring consistent delivery of quality medical devices that meet customer and regulatory expectations.
Answer:
To achieve ISO 13485 certification:
Prepare all company information in a structured way (hiring a consultant is optional but helpful).
Document all relevant processes and QMS procedures.
Implement the documented system across the organization.
Conduct internal audits during and after implementation.
Invite a certification body for auditing.
If your QMS meets the requirements, the certification body will approve and grant the ISO 13485 certificate.
Answer:
The cost varies based on factors such as:
Number of employees
Number of branches
Business size and complexity
QMS maturity
A certification body will assess these factors before offering a quotation.
Answer:
An ISO 13485 certificate is valid for three years. During this period, annual surveillance audits are required to ensure ongoing compliance.
Answer:
To maintain certification:
Undergo annual surveillance audits for three years.
Continually improve and update your QMS.
At the end of the three-year cycle, your organization must undergo recertification to renew the certificate.
Answer:
ISO 13485 certification is suitable for any organization involved in the medical device industry. It helps reduce costs, increase profitability, improve quality control, attract new customers, and retain existing customers by demonstrating commitment to quality and safety.
CERTIFICATION PROCESS
Ready to Get ISO Certified?
Join 500+ Global Companies that have Successfully Achieved ISO Certification with Us.
Missing Something?
We’re here to help you find exactly what you need—just let us know, and we’ll guide you in the right direction.

